Introduction:
Patients (pts) with hematologic malignancies receive more aggressive end-of-life care, including hospitalizations and antineoplastic therapy, compared to solid tumor. 1 In the modern era, treatment for lymphoma and multiple myeloma is mostly administered in the outpatient setting. Pts requiring inpatient treatment may have an aggressive disease course, decreased performance status, and comorbidities that impact clinical outcomes. Treatment decisions in the acute care setting are challenging due to the need to balance efficacy, toxicity, and cost. Given the paucity of data available to help guide decision-making, we sought to investigate the outcomes of inpatient administration of unplanned antineoplastic therapy.
Methods:
This retrospective cohort study included adults (≥18 years) with lymphoma and multiple myeloma who received unplanned inpatient antineoplastic therapy between June and October 2022. Pts who received planned chemotherapy or first cycle of treatment for newly diagnosed disease were excluded. Pts undergoing outpatient induction or salvage therapy admitted for complications who subsequently received their next cycle while admitted were included. Baseline characteristics, diagnosis, treatment, and clinical outcomes were abstracted from the electronic medical record. The primary endpoint was rate of treatment continuation or completion 60 days after first administration of inpatient therapy. Secondary endpoints included overall survival (OS); length of stay (LOS) following treatment; transfer to intensive care unit (ICU), emergency department (ED) visits, and readmissions within 30 days; and death within 30 and 60 days of inpatient treatment.
Results:
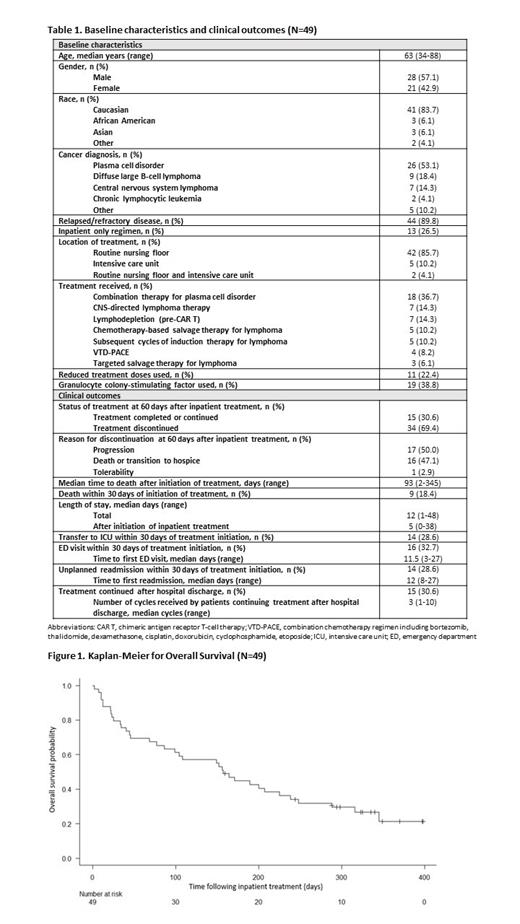
Of the 172 pts screened, 49 pts were included after excluding 122 pts (76 had planned chemo and 46 had newly diagnosed disease). Median age was 63 years (range, 34-88). Treatment was for a variety of diagnoses, including plasma cell neoplasm (53%), diffuse large B-cell lymphoma (18%), central nervous system lymphoma (14%), chronic lymphocytic leukemia (4%), as well as 1 pt each with T-cell prolymphocytic leukemia, follicular lymphoma, mantle cell lymphoma, NK/T-cell lymphoma, and T-cell lymphoma. Most had relapsed/refractory disease (90%). Thirteen (26%) pts received inpatient only regimens; however, these were not planned admissions. Fifteen (31%) pts received drugs traditionally restricted to outpatient use only (daratumumab (10), carfilzomib (3), obinutuzumab (1), loncastuximab tesirine (1), polatuzumab vedotin (1)). At 60 days after first inpatient treatment, 15 (31%) pts continued or completed treatment, while treatment was discontinued in 34 (69%) pts. Of the 15 pts who continued treatment, 53% (8/15) received ≤ 3 additional cycles after discharge. Median time to discharge following inpatient treatment initiation was 5 days (range, 0-38). Fourteen (29%) pts were transferred to the ICU, 16 (33%) pts presented to the ED after discharge, and 14 (29%) were readmitted within 30 days of inpatient treatment. Overall survival was 26.5% at a median follow up of 157 days. The 30- and 60-day mortality rates were 18% and 31% respectively. Univariate and multivariate analysis to identify prognostic factors for survival will be conducted.
Conclusions:
In this retrospective analysis, about a quarter of pts with lymphoid and plasma cell malignancies were alive at 5 months after receiving unplanned inpatient antineoplastic therapy. Better tools are needed to select pts who may benefit from urgent inpatient treatment, as a small subset of our cohort experienced continued clinical benefit. Combining such tools with multidisciplinary discussions may help to maximize favorable outcomes while minimizing use of aggressive end-of-life antineoplastic treatment. Ongoing analysis in our population will aim to identify patient-specific factors associated with positive outcomes.
References:
1. Hui D, Didwaiya N, Vidal M, et al. Quality of End-of-Life Care in Patients with Hematologic Malignancies. Cancer. 2014;120:1572-8.
Disclosures
Rice:Janssen: Other: Advisory Board. Valent:Alexion, AstraZeneca Rare Disease: Research Funding. Williams:Bristol Meyers Squibb: Consultancy; Abbvie: Consultancy; Janssen: Consultancy. Khouri:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; GPCR Therapeutics: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events. Hill:Bristol Myers Squibb: Consultancy; Genentech: Consultancy, Other: Advisory board, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; Pharmacyclics: Consultancy, Other: Advisory board, Research Funding; Incyte: Consultancy; Gilead: Other: Advisory board; BeiGene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Other: Advisory board, Research Funding. Winter:BeiGene: Consultancy; ADC Therapeutics: Consultancy; AstraZeneca: Consultancy; Seattle Genetics: Consultancy; Janssen: Consultancy. Caimi:Genentech: Consultancy; BMS: Consultancy; Novartis: Consultancy; Lilly Oncology: Consultancy; SOBI: Honoraria; ADC Therapeutics: Consultancy; Kite Pharma: Honoraria. Jagadeesh:Affimed: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; Trillium Pharmaceuticals: Research Funding; ATARA Biotherapeutics: Research Funding; Debio Pharma: Research Funding; LOXO Pharmaceuticals: Research Funding; MEI Pharma: Research Funding; Regeneron Pharmaceuticals: Research Funding; Seagen: Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal